Writing Clinical Research Protocols
Writing Clinical Research Protocols. Manoo Bhakta, MD Principal Investigator, COG Associate Professor, UT. Thinking about writing a Protocol?. Protocol- “ …a complete written description of, and scientific rationale for, a research activity involving human subjects.” (Protomechanics NIH)
Share Presentation
Embed Code
Link
Download Presentation
- ctep cancer
- significant risks
- good study design section
- human subject protection
- recruitment
- ancillary procedures

genera + Follow
Download Presentation
Writing Clinical Research Protocols
An Image/Link below is provided (as is) to download presentation Download Policy: Content on the Website is provided to you AS IS for your information and personal use and may not be sold / licensed / shared on other websites without getting consent from its author. Content is provided to you AS IS for your information and personal use only. Download presentation by click this link. While downloading, if for some reason you are not able to download a presentation, the publisher may have deleted the file from their server. During download, if you can't get a presentation, the file might be deleted by the publisher.
Presentation Transcript
- Writing Clinical Research Protocols Manoo Bhakta, MD Principal Investigator, COG Associate Professor, UT
- Thinking about writing a Protocol? • Protocol- “ …a complete written description of, and scientific rationale for, a research activity involving human subjects.” (Protomechanics NIH) • 3 general types of protocols: • 1. Retrospective review- usually with data • 2. Natural History study- may get tissue samples, DNA • 3. Interventional – Phase I/II, Phase III, Phase IV
- If you plan to do clinical research you need a copy of the regulations www.clinicalresearchresources.com
- Writing a Protocol – First steps • As PI, you usually are the one who has an idea • Is it reasonable? Do we have the resources? • Significant risks? • Do you have the patient population? • Include those important to the conduct of the protocol as an Associate Investigator • IND request, outside investigators, COI • Initially most ICs will ask you to write an abbreviated conceptual sheet outlining what you wish to do- to the Pre-IRB committee
- Who Reads Protocols? • Keep the “audience” in mind: • Other physicians • Nurses/CRAs • IRB members • Scientific reviewers
- Using Templates • Many NIH programs encourage or require the use of protocol templates, such as the ones available here: • http://ctep.cancer.gov/guidelines/templates.html • Following template guidelines can help guide authors, but not every part of a template will necessarily apply in a given study.
- Introduction/Abstract Objectives (including study schema) Background/Rationale Eligibility criteria Study design/methods (including drug/device info) Safety/adverse events Regulatory guidance Statistical section (including analysis and monitoring) Human subjects protection/informed consent Parts of the Protocol Adapted from: Protomechanics: Chapter 1 (http://www.cc.nih.gov/ccc/protomechanics/), CTEP Investigators’ Handbook, 2002 (http://ctep.cancer.gov/forms/Hndbk.pdf )
- Objectives • Objectives should be stated clearly as hypotheses to be tested. • Each objective should have a corresponding discussion in the statistical section. CTEP Investigators’ Handbook, 2002 (http://ctep.cancer.gov/handbook/index.html)
- Background and Rationale • All protocols require a section detailing the scientific rationale for a protocol and the justification in medical and scientific literature for the hypothesis being proposed. • Introductory section should be as succinct as possible and should be organized in a logical, sequential flow.
- Background and Rationale • Double check all citations: • “Bibliographic inaccuracies harm the citing author and may cast doubt on the quality of the research being reported…” Wyles DF, Behavioral and Social Sciences Librarian, 2004 • “…[A]uthors should verify references against the original document.” Uniform Requirements for Manuscripts Submitted to Biomedical Journals, ICMJE, 2005 (http://www.icmje.org/)
- Writing Eligibility Criteria STOP BEFORE YOU WRITE! • Eligibility criteria are the largest barrier to accrual to clinical trials.1 • Poorly written or poorly conceived criteria may undermine a trial’s generalizability and scientific validity.2 1Fuks A, J Clin Epidemiol, 19982George SL, J Clin Oncol, 1996
- Writing Eligibility Criteria • Eligibility criteria—stated as either exclusion or inclusion criteria—define and limit the kinds of patients that can participate in a clinical trial. • Reasons for imposing eligibility criteria can include scientific rationales, safety concerns, regulatory issues, and practical considerations. George SL, J Clin Oncol, 1996
- Writing Eligibility Criteria • Problems with restrictive criteria: • Limitations of generalizability • Failure to mimic clinical practice • Increased study complexity • Increased costs • Decreased patient accrual George SL, J Clin Oncol, 1996
- Writing Eligibility Criteria • Recommendations: • The number of eligibility criteria should be kept to a minimum. • Criteria should include only those absolutely necessary to ensure scientific validity and patient safety. • Eligibility criteria should be clearly defined and verifiable by an external auditor. • Adapted from: • George SL, J Clin Oncol, 1996Fuks A, J Clin Epidemiol, 1998
- Writing Eligibility Criteria • Eligibility criteria should be straightforward and unambiguous. Which of these criteria would you choose? • Pregnant and/or nursing women are not eligible. • All women of childbearing age are required to have a serum pregnancy test. • Pregnant and/or nursing women are not eligible for this study. All women of childbearing potential (defined as…) must have a negative pregnancy test (serum or urine) within 2 weeks of study enrollment.
- Writing Eligibility Criteria • However, be aware of the consequences of highly specific criteria: • For example: consider the issues that will follow from mandating a particular serum concentration of some marker, rather than building the definition around institutional upper limits of normal.
- Study Design • The study design section of the protocol should contain a stepwise description of all procedures required by the study. • A good study design section includes sufficient information for the participating site to develop a comprehensive clinical pathway for study patients.
- Study Design • Parts of the study design section may include: • Initial evaluations • Screening tests • Required lab tests • Details of treatment and ancillary procedures • Agent information or device specifications • Dose scheduling and modification • Calendars Adapted from: Protomechanics: Chapter 1 (http://www.cc.nih.gov/ccc/protomechanics/)
- Safety • The Safety (or Adverse Events) section should include: • Detailed information for reporting adverse events, including reporting to the FDA and/or the sponsor • Unblinding processes (if applicable) • Lists of expected adverse events
- Human Subjects Protection • This section includes discussion of: • Subject selection and exclusion • Proposed methods of patient recruitment • Minority representation • Recruitment (or exclusion) of special subjects, including vulnerable subjects • Lists of potential risks and benefits, including justification for risks Adapted from: OHSR Information Sheet 5: Guidelines for Writing Research Protocols (ohsr.od.nih.gov/info/sheet5.html )
- Model Informed Consent • The Office of Human Subjects Research recognizes 3 fundamental conditions for a valid informed consent: • Disclosure of relevant information to prospective research subjects • Comprehension of the information provided • Voluntary agreement of the subject, free from coercion Adapted from: OHSR Information Sheet 6: Guidelines for Writing Informed Consents (ohsr.od.nih.gov/info/sheet6.html )
- Model Informed Consent • To these ends, the protocol model informed consent document must: • Be thorough and complete • Be written in simple, nontechnical language • Be carefully worded to avoid potentially coercive phrasing
- Model Informed Consent • OHSR recommends the following be included: • Statement that the study involves research • Purpose of the research and the length of the study • Description of risks and benefits • Discussion of alternative therapies • Confidentiality policy • Compensation for injury • Contact for further questions/information • Statement of voluntary participation Adapted from: OHSR Information Sheet 6: Guidelines for Writing Informed Consents (ohsr.od.nih.gov/info/sheet6.html )
- Model Informed Consent • Writing a good MIC is a balancing act between being thorough, being accurate, and being as concise and simple as possible. • Patient advocates may offer invaluable experience and insight during the drafting and review phase of an MIC.
- The Statistical Section • Make sure that study objectives and study design elements in the statistical section mirror those in described in the Objectives section! • If the study involves stopping rules, make sure that descriptions and definitions of toxicities in the statistical section match those in the Safety/AE section.
- Good Writing Matters “…Many existing clinical trials contain problems such as incompleteness, ambiguity, and inconsistency. Most of the errors are introduced during the protocol writing process…” Weng C, Medinfo, 2004
- Good Writing Matters • Costs of a badly written protocol? • Poorly written inclusion criteria have resulted in a number of ineligible and inevaluable patients being enrolled to a study. NOW WHAT?
- Good Writing Matters • To fix this problem, the protocol has to be amended. Remember: “Any change to the protocol document or Informed Consent that affects the scientific intent, study design, patient safety, or human subject protection is considered an amendment, and therefore mustbe approved by your IRB…” NCI CTEP Amendment Request Submission Policy, Version Date May 14, 2004 (http://ctep.cancer.gov/guidelines/templates.html)
- Tools for Better Writing: Proofreading • Fresh eyes • Working too long on a protocol may habituate eyes and brains to mistakes, simply because they’ve been there all along. Get an “outside” reviewer! • Spell-checkers, etc. • A document that has been “checked” by automatic software has NOT been proofread.
- NIH Guidance on Protocol Writing • Protomechanics: http://www.cc.nih.gov/ccc/protomechanics/ • The Office of Human Subjects Research: http://ohsr.od.nih.gov/info/info.html • The NCI Investigators’ Handbook: http://ctep.cancer.gov/handbook/index.html
- Templates • The NIH phase III template: http://www.ninds.nih.gov/funding/research/clinical_research/ProtocolTemplate.htm • The Cancer Therapy Evaluation Program (CTEP) Templates (phases I–III; based on NIH model): http://ctep.cancer.gov/guidelines/templates.html
- Informed Consents • Office of Human Subjects Researchhttp://ohsr.od.nih.gov/info/info.html • The Office for Human Research Protections (OHRP): http://www.hhs.gov/ohrp/policy/index.html#informed
- Writing Resources • The International Committee of Medical Journal Editors (ICJME) Uniform Requirements for Manuscripts Submitted to Biomedical Journals http://www.icmje.org/
- Writing Resources • The American Medical Association style manual may provide a useful “default” reference for protocol writing: Cheryl Iverson, Chair. The American MedicalAssociation Manual of Style. 9th ed. Lippincott Williams and Wilkins; 1998. • The Duke University Medical Center Library maintains a select bibliography of style guides at: http://www.mclibrary.duke.edu/subject/style
- Questions?

Writing Clinical Documents
Writing Clinical Documents. Communication Sciences and Disorders. What are clinical documents?. Reports that document what goes on in the clinical setting. Diagnostic reports Progress notes Progress reports Evaluation reports. General Guidelines. Include all necessary information
352 views • 14 slides

Clinical Research
Clinical Research. Methods Observation Unsystematic Controlled Case Studies Each has it’s place…but you should be aware of the limitations of each approach. Research Methods in Psychology.
511 views • 25 slides

PROTOCOLS / CLINICAL PRACTICE GUIDELINES
PROTOCOLS / CLINICAL PRACTICE GUIDELINES. KNR 365. WHAT ARE PROTOCOLS?. Provide link between addressing client needs & evaluating the effects of service delivery Document the purposeful procedures used to deliver intervention to clients
605 views • 30 slides

Clinical Research:
Clinical Research:. Sample Measure (Intervene) Analyze Infer. A study can only be as good as the data . . . -J.M. Bland i.e. no matter how brilliant your study design or analytic skills you can never overcome poor measurements. .
742 views • 49 slides

Clinical Research
2. Agenda. Site Information and CDA Process Review Potential Patients Regulatory 1572 CVs and License Record Financial Disclosures Delegation of responsibilities Study Budget and Negotiation Letters of Support Contract (OSP) IRB Submission and OSP Routing Form Re
575 views • 39 slides

CLINICAL REPORT WRITING
CLINICAL REPORT WRITING. In Child and Youth Work Nancy Brown Brunton. General Pointers. All of your notes, regardless of format, are legal documents. Most agencies prefer black pen for all written work; never use pencil. All written work must be professionally written. CONFIDENTIALITY.
224 views • 8 slides

IRB Review of Multi-Site Pediatric Clinical Research Protocols
IRB Review of Multi-Site Pediatric Clinical Research Protocols. Steven Hirschfeld, MD PhD Captain, U.S. Public health Service Associate Director for Clinical Research Eunice Kennedy Shriver National Institute of Child Health and Human Development
487 views • 30 slides

Clinical research
Clinical research. Cranfield University . Alcohol, alcoholism and withdrawal. Shovan K B ICRI, Cranfield University.
562 views • 33 slides

Clinical research
Clinical research. Cranfield University . Drug abuse & Drug dependence. Shovan K B ICRI, Cranfield University. Drug . Early 14 th century, old French word “ Drouge ”. May be from old/middle Dutch “ Droog ”= dry, arid. association with "poisons" is 1500s.
447 views • 28 slides

Writing Protocols in OCL
Writing Protocols in OCL. CS 4311 Jos B. Warmer and Anneke G. Kleppe, OCL: The Constraint Language of the UML, JOOP , May 1999. Jos B. Warmer and Anneke G. Kleppe, The Object Constraint Language, second edition, Addison-Wesley, 2003. 1. 1. Outline. Motivation Basics of OCL
457 views • 27 slides

Clinical Note Writing
Clinical Note Writing. Pharmacy Department Dale Tucker, RPh, BCPS Elizabeth Cincotta, PharmD Detroit Medical Center Last Updated July 2005 by Julie Berman (DRH), Albert Bajjoka (HVSH), May Saba (CHM), Kim Tsilimingras (SGH), & Dale Tucker (HUH). Goals and Objectives.
463 views • 24 slides

Writing Clinical Research Protocols Part 1: Preparation and Process
Writing Clinical Research Protocols Part 1: Preparation and Process. Jonathan McCall, BA Editor DCRI Communications. The Protocol-writing Process. The Protocol-writing Process. Clinical Protocols: an Overview Preparatory Steps Assembling a Protocol Team Managing the Process
776 views • 39 slides

Writing Clinical Research Protocols, Part 2: Writing and Editing Clinical Research Protocols
Writing Clinical Research Protocols, Part 2: Writing and Editing Clinical Research Protocols. Jonathan McCall, BA Editor DCRI Communications. Writing Clinical Research Protocols. Writing Clinical Research Protocols. General Considerations Parts of the Protocol Good Writing Matters
757 views • 51 slides
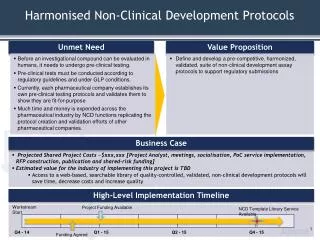
Harmonised Non-Clinical Development Protocols
High-Level Implementation Timeline. Business Case. Unmet Need. Value Proposition. Harmonised Non-Clinical Development Protocols.
168 views • 1 slides

Protocols and clinical methodology
Protocols and clinical methodology. Paul W. Jones Professor of Respiratory Medicine St George’s Hospital Medical School London, UK. Purpose of cardiopulmonary exercise tests. Measure work (exercise) capacity Investigate breathlessness Measure effect of treatment.
427 views • 34 slides

Writing Protocols in OCL
Writing Protocols in OCL. CS 4311 Jos B. Warmer and Anneke G. Kleppe, OCL: The Constraint Language of the UML, JOOP, May 1999. Jos B. Warmer and Anneke G. Kleppe, The Object Constraint Language: Precise Modeling with UML, Addison-Wesley, 1998. 1. 1. Outline. Motivation Basics of OCL
313 views • 22 slides

Writing Clinical Notes
Writing Clinical Notes. Writing in CSD. Physical characteristics of Clinical Notes. Brief Describe client’s response to objectives Recommendations for the next session. Being objective. “Report what you observe, not what you think!”. Objective/Goal Format. Client + target + criterion
231 views • 10 slides

Clinical Writing/ Records
Clinical Writing/ Records. Presentation By: Ben Curll. What is Clinical Writing anyway?. clinical writing is a form of record keeping for health/ mental health professions used to document client what their current situation is progress of client in therapy . Good Clinical Writing.
283 views • 11 slides

Adaptable Clinical Pathways and Protocols
Adaptable Clinical Pathways and Protocols. HCLS F2F Meeting Amsterdam, The Netherlands Oct. 3-4, 2006. Charters. A descriptive mechanism that expresses the tasks involved in protocol and process definition.
208 views • 6 slides

Clinical Research Courses, Clinical Research Institute
There are all kinds of Clinical Research Courses are available in our institute. for more details of clinical research visit at : http://icri-india.com
247 views • 8 slides

Clinical Research Courses, Clinical Research Institute
An institute of clinical research helps people in making bright careers in this particular field. Visit at : http://icriindia.com/
201 views • 4 slides

clinical research
Clinical Scoop is a leading pioneer of the major discoveries and innovations in the field of medicine. A significant number of research experts and medical practitioners are associated with this firm who are working tirelessly to figure out exceptional ways to introduce groundbreaking health remedies
























